12 R723 – an ammonia-based azeotrope // R723 – ein Azeotrop auf der Basis von Ammoniak
Alongside the refrigerant, oil is a key operating material in refrigeration systems. In a closed circuit, the oil needs to circulate back to the compressor. At low temperatures, oils become viscous or may even reach their pour point and become solid. The refrigeration sector has looked to address this problem by using refrigeration oils which are highly miscible with the refrigerant. This reduces the viscosity of the oil significantly.
In the 1990s, soluble oils based on polyalkylene glycol were developed for ammonia and have now been successfully used in many systems. However, there have also been increasing problems with the refrigeration cycle’s chemical stability. These problems are mainly caused by the oil’s high hygroscopicity, which may result in full miscibility with water. With the added factor of high temperatures and high moisture levels, this can make the refrigeration cycle unstable, especially if the system contains aluminium materials. This instability may then cause the refrigeration system to break down.
For this reason, tests were performed during which a gaseous substance was added to ammonia as a ‘solubility agent’ to achieve effective oil solubility with conventional mineral oils. The first successful tests were performed using amine/ammonia mixtures, which significantly improved oil behaviour but had disadvantages for the refrigeration cycle, especially in heat exchangers, due to their marked non-azeotropic behaviour.
Dimethyl ether, a solvent widely used in the chemical industry, was also studied for its suitability as an additive. It is used as propellant in many sprays and is similar to isobutane when used as a refrigerant. When its miscibility behaviour with ammonia was studied, findings unexpectedly showed that the two substances form an azeotrope. The concentration of ammonia in the azeotrope is 60 mass percent. This means that the mixture can be treated as a pure substance within this concentration range. Consequently, there is no change in concentration during condensation and evaporation or during leaks. Since the molar mass of the mixture is 23, it was designated the code R-723 in line with the refrigerant numbering system for natural refrigerants. However, R-723 is not the official number designated by ISO 817, although it has now become the established code used by many refrigeration professionals.
This mixture is not yet officially listed in DIN EN 378 since it first needs to undergo a complex registration procedure and be issued an R number.
Besides its improved oil solubility, regarded as the initial decisive factor, R-723 has a number of other positive properties. These have been studied in a number of projects and have also been verified in various field trials.
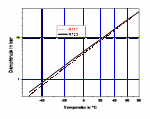
Figure 1 shows the mixture’s vapour pressure compared to ammonia. You can see that its vapour pressure almost matched that of ammonia and is only somewhat higher at low temperatures.
Figure 1: Vapour pressures of R-717 and R-723 in relation to temperature
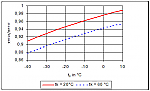
This means that the compression ratio is lower at the same evaporation and condensing temperatures, as can be seen in Figure 2.
Figure 2: Relative pressure ratio
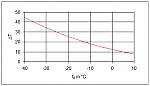
As the mixture’s adiabatic exponent is lower due to the higher molar mass, a lower compression temperature must also be expected (Figure 3).
Figure 3: Difference in the discharge temperature between ammonia and R-723 at a condensing temperature of 40 °C
The greater volumetric refrigeration capacity and also the higher COP values in comparison to ammonia are noteworthy. This difference to pure ammonia deserves special attention in TEWI assessments of refrigeration systems. The COP value has improved by 7% in refrigeration systems initially operated with ammonia and subsequently with R-723.
It should also be pointed out that using mineral oils with the mixture achieves considerable increases in heat transfer coefficients during dry evaporation thanks to the use of the mixture.
The data on chemical media for R-723 are already included in the chemical media data programmes for ASERCOM.
If only its lower explosion limit of 6.0 volume percent were considered, the mixture would belong to Group B2 as per DIN EN 378 (toxic and flammable), the same as ammonia. It would thus be treated in the same way as ammonia from a safety perspective. However, the second parameter relevant to classification, its specific combustion heat (i.e. the energy released on burning only), is 22,800 kJ/kg, which is above the limit value of 19,000 kJ/kg arbitrarily specified for Group B2. As a result, it would be classified in Group B3, in terms of flammability belonging to the same Group (A3) as R-290 (propane).
Limits are often imposed on ammonia charge for safety reasons. Better refrigeration capacities can be achieved with R-723 compared to the same charge with ammonia.
Materials that are suitable for ammonia can also be used in the mixture. There is only a need to test for sufficient resistance to dimethyl ether if elastic polymers are used as sealants in case they come into direct contact with the product. Many major component manufacturers tested the product at an early stage. They have been able to offer a range of components necessary for refrigeration systems using R-723 as a working fluid for some time now.
R-723 can be used primarily in new systems, even those with smaller refrigerating capacities far below 100 kW, since the circulating mass flow in the vapour phase is about 150% compared to ammonia. This means pipe gauges and flow velocities are acceptable, even for smaller refrigerating capacities.
The refrigerant has been commercially available for a number of years and can be obtained from selected refrigerant dealers in Germany.
Further publications:
Steiner, A., Lippold, H.: Umstellung einer Ammoniak-Kälteanlage auf das Gemisch NH3/Dimethylether (Converting an ammonia refrigeration system to NH3/dimethyl ether
Seminar: Stand und Anwendung natürlicher Kältemittel (Status and use of natural refrigerants); Mainz 2002.
Lippold, H., Heide, R.: Dimethylether als Kältemittelkomponente (Dimethyl ether as a refrigerant component) KI- Luft- und Kältetechnik 33 (1997) 5, p.202–205.
Lippold, H.: Wärmeübergangskoeffizienten bei der Verdampfung von NH3 und NH3-Dimethylether-Gemisch (Thermal transmittance coefficients during evaporation of NH3 and an NH3/dimethyl ether mixture) KI- Luft- und Kältetechnik 37 (2001) 2, p.78–82.
Lippold, H., Vollmer, D.: Stoffdatenprogramm für Kältemittel (Chemical media data programme for refrigerants) KI- Luft- und Kältetechnik 34 (1998) 3, p.141.
Krauss, D.: Fragen und Antworten rund um das Kältemittel ‘schickR723®’ (Questions and answers about the refrigerant ‘schickR723®’) Kälte Klima Aktuell 1/2009, p.26–31.
Krauss, D., Schenk, J.: Use of Ammonia/Dimethyl Ether (schick R723®) Blend in Medium Refrigeration Plants
Proc. Int. Conf. “Ammonia Refrigerating Technology for Today and Tomorrow”
Ohrid, Macedonia, 19–21 April, 2007, ISBN 987-2-913149-57-1, www.mf.ukim.edu.mk
J. Germanus, S. Römer, D. Krauss: Applications of schickR723® Blend in Commercial Refrigeration Systems; Proc. Int. Conf. “Ammonia Refrigerating Technology for Today and Tomorrow” Ohrid, Macedonia, 6–9 May, 2009, www.mf.ukim.edu.mk
Information brochure, Kälte Concept GmbH, www.kaelteconcept.de
Künftigen Auflagen einen Schritt voraus (A step ahead of future requirements)
KI- Luft- und Kältetechnik 09 (2008) 33, p.33–34